A free import export trade leads B2B marketplace since 1996 for manufacturers, suppliers, exporters, importers, buyers, sellers, wholesalers, freight forwarders, shippers, trade shows, exhibits, and export management.
Hello visitor from USA - 47 visiting from
USA - 47 visiting from  25 USA
25 USA  14 Canada
14 Canada  3 UK
3 UK  1 India
1 India  1 Iran
1 Iran  1 Argentina
1 Argentina  1 China
1 China  1 Costa Rica
1 Costa Rica
Hello visitor from
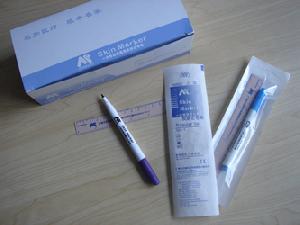
Surgical Marking Pen
Posted at: Offers to Sell and Export | Posted on: Friday 3 August 2007 3:40 am | Poster last visit: Monday 5 March 2012 |
Product Category: Main
» Tools and General Machinery
» Hand tools
» Marking tools
» Marking pen
» Surgical Marking Pen
Product Description:
1. Skin marker has obtained FDA, CE, ISO13485 and ISO9001Certificate and we undertake OEM orders;
2.
The ink of skin marker is certificated by CE and tested for Biocompatibility Test by independent laboratory, the markers can be used safely without cytotoxicity, irritation response to skin and contact sensitization;
3. Skin marker is a medical device and doesnít belong to stationery and toy, meets medical device standard and regulation;
4. Skin marker with a ruler is gamma radiation sterilized;
5. The ink can paste strongly on the skin and not easy to be erased in cleaning and disinfection;
6. Both formulation and package ensure the validity of ink and canít be dried up in normal use
and without dry;
7. We usually use two kinds of skin markers in clinical, they are regular type
(with 1 mm broad line) and fine type (with 0.5 mm broad line);
8. Sterilized products can meet the single use requirement and avoid infection;
Company Contact:

Contact Name: Mr. Pan
Company Name: Medplus Inc.
Email:
MSN:
Tel: +86- 20-34773301-608
Fax: +86-20-34772221
Street Address: 4th Floor, BuildingC-4,
Gaosha Industrial Zone, Zhongcun, Panyu
District, Guangzhou, 511495, P.R. China
Website: https://www.gzmedplus.com
Member name: plus3477
Country: China
China
Member Since: 18 June 2007
Total Leads: 8 plus3477 Import Export Business Leads
Business focus: Surgical Skin Marker, Loss Of Resistance Syringe, Breathing Circuit, Urethral Catheter, Tracheal Tubes, Biopsy Kits
Chat:
Verify: Safe Import Export Tips
2.
The ink of skin marker is certificated by CE and tested for Biocompatibility Test by independent laboratory, the markers can be used safely without cytotoxicity, irritation response to skin and contact sensitization;
3. Skin marker is a medical device and doesnít belong to stationery and toy, meets medical device standard and regulation;
4. Skin marker with a ruler is gamma radiation sterilized;
5. The ink can paste strongly on the skin and not easy to be erased in cleaning and disinfection;
6. Both formulation and package ensure the validity of ink and canít be dried up in normal use
and without dry;
7. We usually use two kinds of skin markers in clinical, they are regular type
(with 1 mm broad line) and fine type (with 0.5 mm broad line);
8. Sterilized products can meet the single use requirement and avoid infection;
Company Contact:

Contact Name: Mr. Pan
Company Name: Medplus Inc.
Email:
MSN:
Tel: +86- 20-34773301-608
Fax: +86-20-34772221
Street Address: 4th Floor, BuildingC-4,
Gaosha Industrial Zone, Zhongcun, Panyu
District, Guangzhou, 511495, P.R. China
Website: https://www.gzmedplus.com
Member name: plus3477
Country:
Member Since: 18 June 2007
Total Leads: 8 plus3477 Import Export Business Leads
Business focus: Surgical Skin Marker, Loss Of Resistance Syringe, Breathing Circuit, Urethral Catheter, Tracheal Tubes, Biopsy Kits
Chat:

Verify: Safe Import Export Tips
 Similar Suppliers And Manufacturers Import Export Trade Leads
Similar Suppliers And Manufacturers Import Export Trade Leads
 Medical Surgical Instruments Parts And Surgical Tools Parts - By Yujiaxin - On Friday 17 March 2023 3:37 am: surgical instruments parts and surgical tools parts
our products are mainly produced by four processes including casting powder metallurgy numeric....
Medical Surgical Instruments Parts And Surgical Tools Parts - By Yujiaxin - On Friday 17 March 2023 3:37 am: surgical instruments parts and surgical tools parts
our products are mainly produced by four processes including casting powder metallurgy numeric.... Fiber Laser Marking Machine St20fd-h - By sequoyatec - On Wednesday 19 October 2022 3:23 am: marking machine st20fd-h
fiber laser cutting machine features †
hand-held design it can marking on the immovable material †
high speed with galva....
Fiber Laser Marking Machine St20fd-h - By sequoyatec - On Wednesday 19 October 2022 3:23 am: marking machine st20fd-h
fiber laser cutting machine features †
hand-held design it can marking on the immovable material †
high speed with galva.... Sell Fiber Laser Marking Machine - By lasermarkingmachine - On Tuesday 17 May 2022 9:09 am: marking machines laser cutter laser welder and laser cleaning machine
and for fiber laser marker we can provide co2 laser marking machine uv las....
Sell Fiber Laser Marking Machine - By lasermarkingmachine - On Tuesday 17 May 2022 9:09 am: marking machines laser cutter laser welder and laser cleaning machine
and for fiber laser marker we can provide co2 laser marking machine uv las.... Hand Painted Pen Holders - By fldchristmasball - On Wednesday 5 October 2022 2:25 am: pen holders
four schools of chinese inside painting art
now days inside painting art has four schools they are jing school lu school ji school an....
Hand Painted Pen Holders - By fldchristmasball - On Wednesday 5 October 2022 2:25 am: pen holders
four schools of chinese inside painting art
now days inside painting art has four schools they are jing school lu school ji school an.... Well Kleannon Woven Surgical Mask Astm Level - By wkmedcare - On Monday 6 June 2022 6:02 am: surgical face mask wholesale from china
product information
wk s surgical face mask is fda cleared manufatured using the highest quality of china m....
Well Kleannon Woven Surgical Mask Astm Level - By wkmedcare - On Monday 6 June 2022 6:02 am: surgical face mask wholesale from china
product information
wk s surgical face mask is fda cleared manufatured using the highest quality of china m.... Natural Rattan Bamboo Woven Pen Holder Wholesales Made In Vietnam Hp H012 - By handipassion - On Tuesday 4 July 2023 9:41 am: natural rattan bamboo woven pen holder wholesales made in vietnam hp h012
size customized
color natural or customized
material rattan and bamboo are woven together
packing paper polybag or gift box packed in 3-5-7 ....
Natural Rattan Bamboo Woven Pen Holder Wholesales Made In Vietnam Hp H012 - By handipassion - On Tuesday 4 July 2023 9:41 am: natural rattan bamboo woven pen holder wholesales made in vietnam hp h012
size customized
color natural or customized
material rattan and bamboo are woven together
packing paper polybag or gift box packed in 3-5-7 .... Astm F67 Gr2 Medical Titanium Bars For Surgical Implants - By tlktitanium - On Monday 3 October 2022 12:18 pm: astm f67 gr2 medical titanium bars for surgical implants
1 name medical pure titanium bars rods
2 standard astm f67
3 material gr1 †gr2 †gr3 †gr4 †
4 size dia2 5 200mm* l
5 moq 50kg
6 shipm....
Astm F67 Gr2 Medical Titanium Bars For Surgical Implants - By tlktitanium - On Monday 3 October 2022 12:18 pm: astm f67 gr2 medical titanium bars for surgical implants
1 name medical pure titanium bars rods
2 standard astm f67
3 material gr1 †gr2 †gr3 †gr4 †
4 size dia2 5 200mm* l
5 moq 50kg
6 shipm.... Hcm Medica 5w Surgery Headlamp Surgical Dental Ent Medical Led Headlight - By HCMMEDICA - On Friday 10 March 2023 7:50 am: hcm medica 5w surgery headlamp surgical dental ent medical led headlight
hcm medica is a professional manufacturer for all kinds of medical headlight product is widely use in ent gynecology neurosurgery cardiosurger....
Hcm Medica 5w Surgery Headlamp Surgical Dental Ent Medical Led Headlight - By HCMMEDICA - On Friday 10 March 2023 7:50 am: hcm medica 5w surgery headlamp surgical dental ent medical led headlight
hcm medica is a professional manufacturer for all kinds of medical headlight product is widely use in ent gynecology neurosurgery cardiosurger.... Import Export Startup Stories
Import Export Startup Stories
Share Your Story & Get Listed at StartImportExport.com- LeBord Surgical Instruments: Arshed Chughtai Of LeBord Surgical Instruments From Pakistan
- I.S. International: Waqas Ishaq Of IS International, A Manufacturer Of Quality Medical, Surgical, Dental, And Orthopedic Instruments From Pakistan
- Wet Metal Surgical: Mustafa Kaal Of Wet Metal Surgical, A Manufacturer Of Surgical, Orthopedic, Dental and Micro instruments From Pakistan
- Fraztys Inc: Fraz Khalid Of Fraztys Inc A Manufacturer Exporter of Surgical And Dental Instruments From USA
 Marking pen Classification Navigation
Marking pen Classification Navigation
Main - Tools and General Machinery - Hand tools - Marking tools - Marking pen [1]
TradersCity.com shall not be held liable for any user posted/submitted content including but not limited to trade leads, profiles, images, and any other data. TradersCity.com does not and did not verify any of users posted/submitted data nor is implicitly or explicitly recommending these business offers. TradersCity does not verify truthfulness, accuracy, completeness, nor legality of any businesses, services, and leads posted here. TradersCity does not represent Sellers or Buyers in any transaction between users of the website and is unable to make any opinion in regard to their performance in any transaction. TradersCity neither guarantees nor undertakes in any dispute between sellers and buyers. Protect your business from fraud by trading safely